What Do We Know?
For convenience, Ka and Kb values can also be expressed on a logarithmic scale:
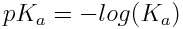 and
and
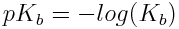
An acid with a small pKa value corresponds to a large Ka value, which indicates that a fairly large amount of acid ionizes in aqueous solution. This tells us that the acid in question is a relatively strong weak acid. Similarly, a small pKa value for a base corresponds to a high Kb value and a large extent of ionization in aqueous solution, indicating a relatively strong weak base.
Below is a table expressing the Ka, Kb, pKa and pKKb values for several weak acids and bases.
| Acid | Ka | pKa | Base | Kb | pKa |
|---|---|---|---|---|---|
| Hydronium ion (H3O+) | 1.0 | 0 | Hydroxide ion (OH-) | 1.0 | 0 |
| Phosphoric acid (H3PO4) | 7.5 × 10-3 | 2.12 | Phosphate ion (PO43-) | 2.8 × 10-2 | 1.55 |
| Acetic acid (CH3COOH) | 1.8 × 10-5 | 4.75 | Carbonate ion (CO32-) | 2.1 × 10-4 | 3.68 |
| Carbonic acid (H2CO3) | 4.2 × 10-7 | 6.38 | Ammonia (NH3) | 1.8 × 10-5 | 4.75 |
| Ammonium ion (NH4+) | 5.6 × 10-10 | 9.25 | Hydrogen Carbonate ion (HCO3-) | 2.4 × 10-8 | 7.62 |
| Hydrogen Carbonate ion (HCO3-) | 4.8 × 10-11 | 10.32 | Acetate ion (CH3COOH) | 5.6 × 10-10 | 9.25 |
| Water (H2O) | 1.0 × 10-14 | 14.00 | Water (H2O) | 1.0 × 10-14 | 14.00 |
When an acid or base reacts with water, chemists measure the concentrations of hydronium and hydroxide ions at equilibrium. These measurements indicate the extent of the reaction and enable chemists to calculate the acid ionization constant (Ka) or base ionization constant (Kb) of the reaction.
Based on the table above, which acid is the strongest? Which base is the strongest? How can you tell?
Hydronium ion is the strongest acid because it has the largest Ka value. Hydroxide ion is the strongest base because it has the largest Kb value.