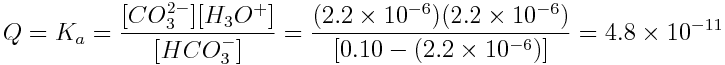Worked Example
Increasing atmospheric carbon dioxide concentrations are changing the balance of equilibria involving the various carbonate species in the ocean, resulting in a decrease in the pH of the world’s oceans. A 0.10 mol L-1 aqueous solution of hydrogen carbonate (HCO3-) at 25 °C has a pH of 5.66. What is the Ka for hydrogen carbonate at 25°C?
Ka can be calculated by putting the equilibrium concentrations of all the reactant and product species into the reaction quotient expression. We can use the solution pH to determine the equilibrium concentration of H3O+(aq) ions and then the equilibrium concentrations of the other species can be derived using stoichiometric principles.
The equation for the ionization of hydrogen carbonate ions in aqueous solution is:
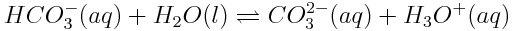
Using the pH of the solution, the equilibrium concentration of H3O+(aq) ions is:
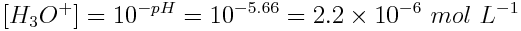
The chemical equation of the reaction shows tells us that if [H3O+] increased by 2.2×10-6 mol L-1, [CO32-] increased by the same amount and [HCO3-] decreased by this amount. Therefore, the equilibrium concentrations of these substances are:
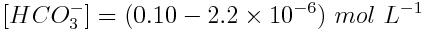
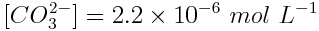

Substitute these concentrations into the equilibrium quotient expression. In this case, because the above concentrations occur when this reaction reaches equilibrium, Q=Ka.