What Do We Know?
Chemists have developed a method of determining the strengths of acids that is more powerful than measuring hydronium ion concentrations of acidic solutions. The strength of an acid depends on the extent to which the acid reacts with water. Therefore, chemists quantitatively express the strength of a weak acid through the equilibrium constant, K, of the ionization reaction between the acid and water. This K value is called the acid ionization constant (Ka). The general reaction between a weak acid (HA) and water is as follows:
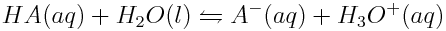
Based on the reaction scheme shown above, the general formula to determine the acid ionization constant, Ka, of the ionization of a weak acid in water at equilibrium is:
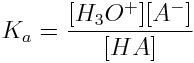
The acid ionization constant provides a convenient means of quickly determining the strength of a weak acid. If an acid has a large Ka, the acid produces a large amount of hydronium ions in solution and is a relatively strong acid.