What Do We Know?
The temperature begins to increase again in the stratosphere due to the presence of ozone. The "ozone layer" is not really a layer, but simply a region which has a concentration of ozone that is slightly higher than the ozone concentration that is found in surrounding regions. Stratospheric ozone contributes to the temperature profile reversal because a cycle of reactions involving ozone, oxygen and sunlight converts ultraviolet radiation (UV) from the Sun into increased kinetic energy of the stratospheric molecules (and hence higher temperature). These reactions are called the Chapman cycle. As altitude increases, the rate of these reactions increases so that, as you move up the stratosphere, more of the energetic molecules are produced and the temperature increases further.
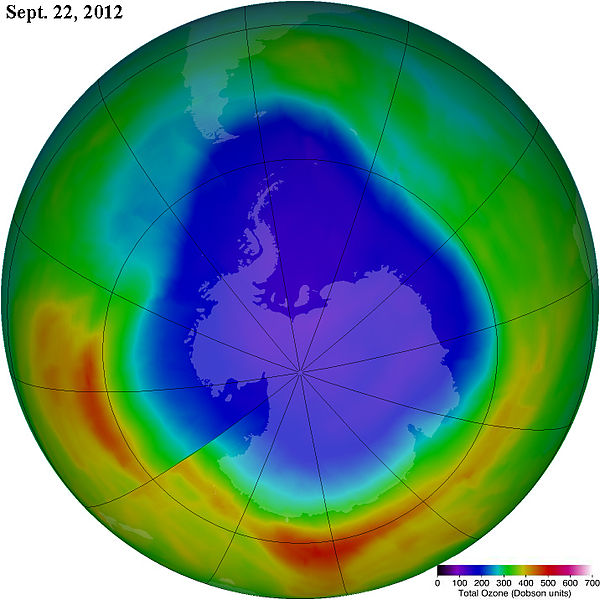
The reaction between ozone and sunlight in the stratosphere is important because it prevents UV radiation from reaching the Earth by converting the UV radiation into increased kinetic energy of the stratospheric molecules. UV radiation has been linked to skin cancer and can damage DNA. If these energy releasing reactions in the stratosphere did not occur, the high levels of UV radiation reaching the Earth's surface would cause severe damage to living beings. Over the past 50 years, human activity has led to the production and release of various substances into our atmosphere. These substances include chlorofluorocarbons, which have increased the rate at which ozone is broken down in the stratosphere, thus allowing more UV radiation to reach the surface. But it is important to keep straight the regions in our atmosphere where this ozone depletion chemistry takes place (the stratosphere) and the processes and regions of our atmosphere where warming occurs due to the presence of greenhouse gases (the troposphere).