Worked Example
Although household furnaces typically use natural gas (methane, CH4(g)), other fuels can also be used, including heating oil and propane (C3H8(g)). Heating oil consists of numerous hydrocarbons that contain 14-20 carbon atoms. We will approximate the molecular formula of heating oil to be C18H38(l). Use the standard molar enthalpy changes of formation given below to calculate the enthalpy change that occurs when one kilogram of each of these fuels is burned in a furnace. Per kilogram, what fuel provides the most energy as heat to warm the house?






Refer to your general chemistry textbook for a thorough discussion on how to use standard molar enthalpy changes of formation to calculate the enthalpy change of a reaction.
Natural gas: First, write the balanced combustion reaction for methane.

Next, calculate the enthalpy change for the reaction.
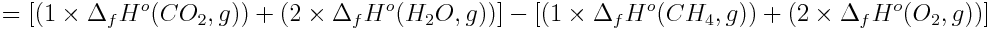


Use the molar mass of methane to determine the enthalpy change per gram and then calculate the enthalpy change when 1 kg (1000 g) of natural gas is combusted.

Heating oil: Write and balance the combustion reaction for C18H38(l).




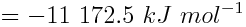
Propane: Write the balanced combustion reaction for propane.





Determine the enthalpy change per gram using the molar mass of propane and then calculate the enthalpy change when 1 kg of propane is combusted.

The enthalpy change of combustion of 1 kg of natural gas is greater than the enthalpy change of combustion of 1 kg of either heating oil or propane. Therefore, combustion of natural gas at constant pressure transfers the greatest amount of energy as heat.