What Do We Know?
What influences the speciation of an element? It is the surrounding environment that determines the speciation of an element. Just as the increasing concentration of dissolved carbon dioxide in the ocean results in a greater production of hydronium ions in the ocean, the speciation of carbon is changed when reactions involving these carbon species are disturbed by changes in environmental factors that influence a particular reaction.
Species of an element are interconverted through chemical reactions, some of which involve multiple equilibrium processes. Therefore, in order to change the speciation of an element, the reactions involving these species must be disturbed.
The change in the speciation of an element in response to a disturbance is based on the values of the reaction quotient and equilibrium constants of reactions involving various species of an element. Remember that reactions shift in response to changes in the concentration of a product or reactant and changes in temperature. Consider the reaction of carbonic acid and water that produces hydrogen carbonate ions:
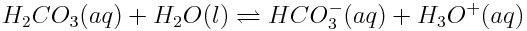
If this reaction is disturbed, either the reaction quotient, Q, or the equilibrium constant, K, will change. When this occurs, Q≠K and the system is no longer at equilibrium. In response, the reaction will shift to restore equilibrium, changing the distribution of carbon between these two species.