What Do We Know?
One way to disturb reaction systems that involve acids and bases is to change the pH. The equilibria of acids and bases involve hydronium or hydroxide ions. Therefore, if a substance added to a solution changes the pH of the solution, the speciation of acids and bases already in the solution will be affected. For example, consider the equilibrium involving carbonic acid and hydrogen carbonate ions:
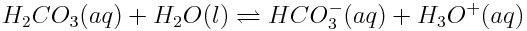
Imagine some HCl(aq) was added to this system. How would the equilibrium be affected?
First of all, the HCl(aq) would ionize and produce hydronium ions. This would increase the concentration of hydronium ions in the solution. Increasing the concentrations of the products causes the value of Q to increase, so that Q≠K.
In order to reach equilibrium, the reaction must shift to reduce Q, so that Q approaches K. To reduce Q, the reaction shifts to the left, converting products to reactants. This causes the concentration of carbonic acid to increase and the concentration of hydrogen carbonate ions to decrease. As H2CO3 only exists temporarily before breaking down into CO2 and H2O, the change in equilibrium could be experimentally verified by the production of CO2 gas.